Description
Ammonium iron(II) sulfate, or Mohr’s salt, is the inorganic compound. Containing two different cations, Fe2+ and NH4+, it is classified as a double salt of ferrous sulfate and ammonium sulfate. It is a common laboratory reagent. Like the other ferrous sulfate salts, ferrous ammonium sulfate dissolves in water to give the aquo complex [Fe(H2O)6]2+, which has octahedral molecular geometry. Ferrous ammonium sulfate hexahydrate is a hydrate that is the hexahydrate form of ferrous ammonium sulfate. Acts as an iron ion donor for building Fe-S clusters in vitro. It is a hydrate and an iron molecular entity.
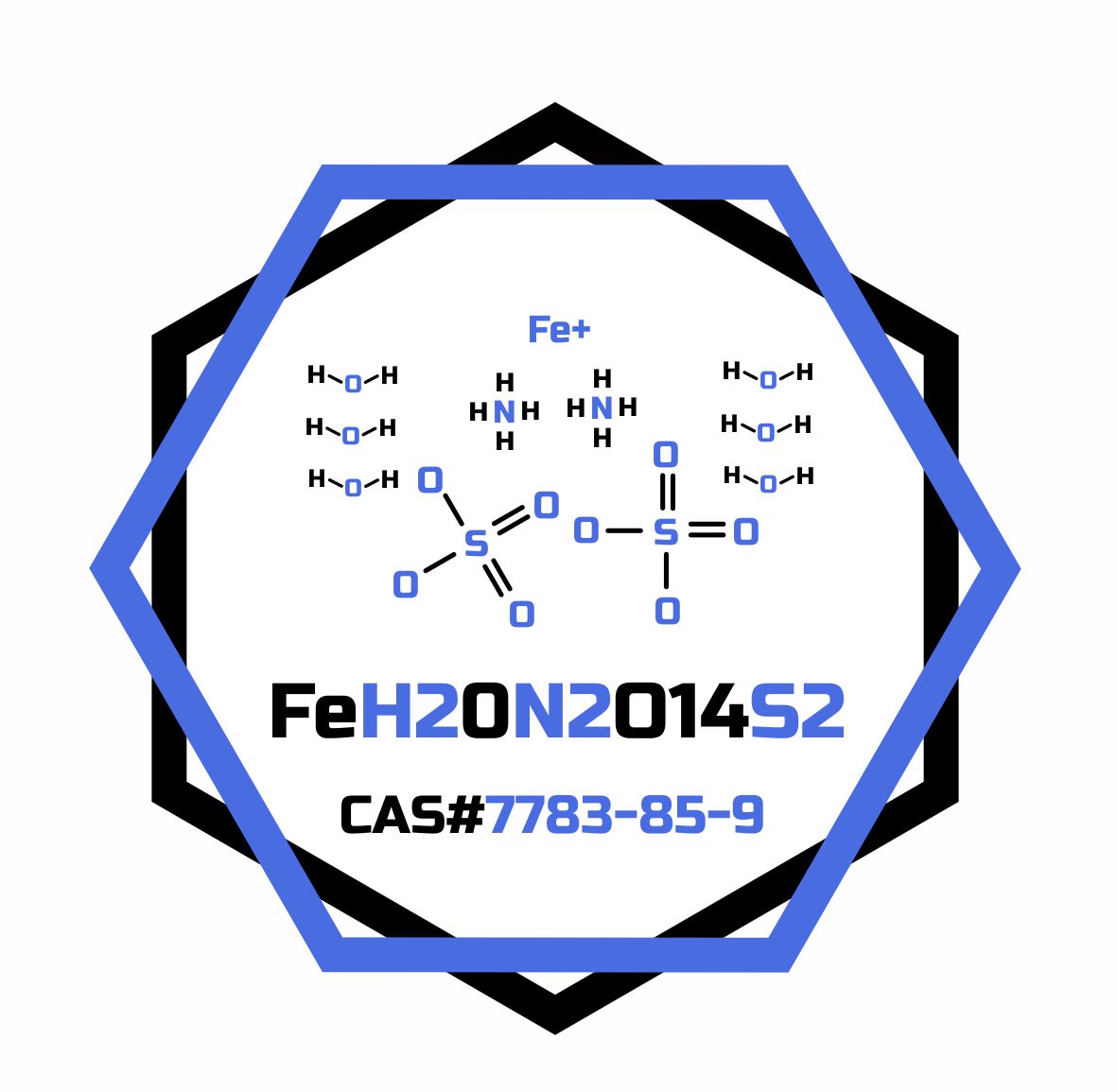
- Formula
- FeH20N2O14S2
- Molar mass
- 392.125 g/mol
- CAS Number
- 7783-85-9
- Density
- 1.86 g/cm3
- Purity/Grade
- 99% (ACS Reagent)
- Apearance
- Solid
- Melting point/freezing point
- 100°C (212°F) – dec.
Reviews
There are no reviews yet.