Description
Monoammonium phosphate is soluble in water and crystallizes from it as the anhydrous salt in the tetragonal system, as elongated prisms or needles. It is practically insoluble in ethanol. Solid monoammonium phosphate can be considered stable in practice for temperatures up to
200°C, when it decomposes into gaseous ammonia NH3 and molten phosphoric acid H3PO4. At 125°C the partial pressure of ammonia is 0.05 mm Hg. Monoammonium phosphate is industrially prepared by the exothermic reaction of phosphoric acid and ammonia.
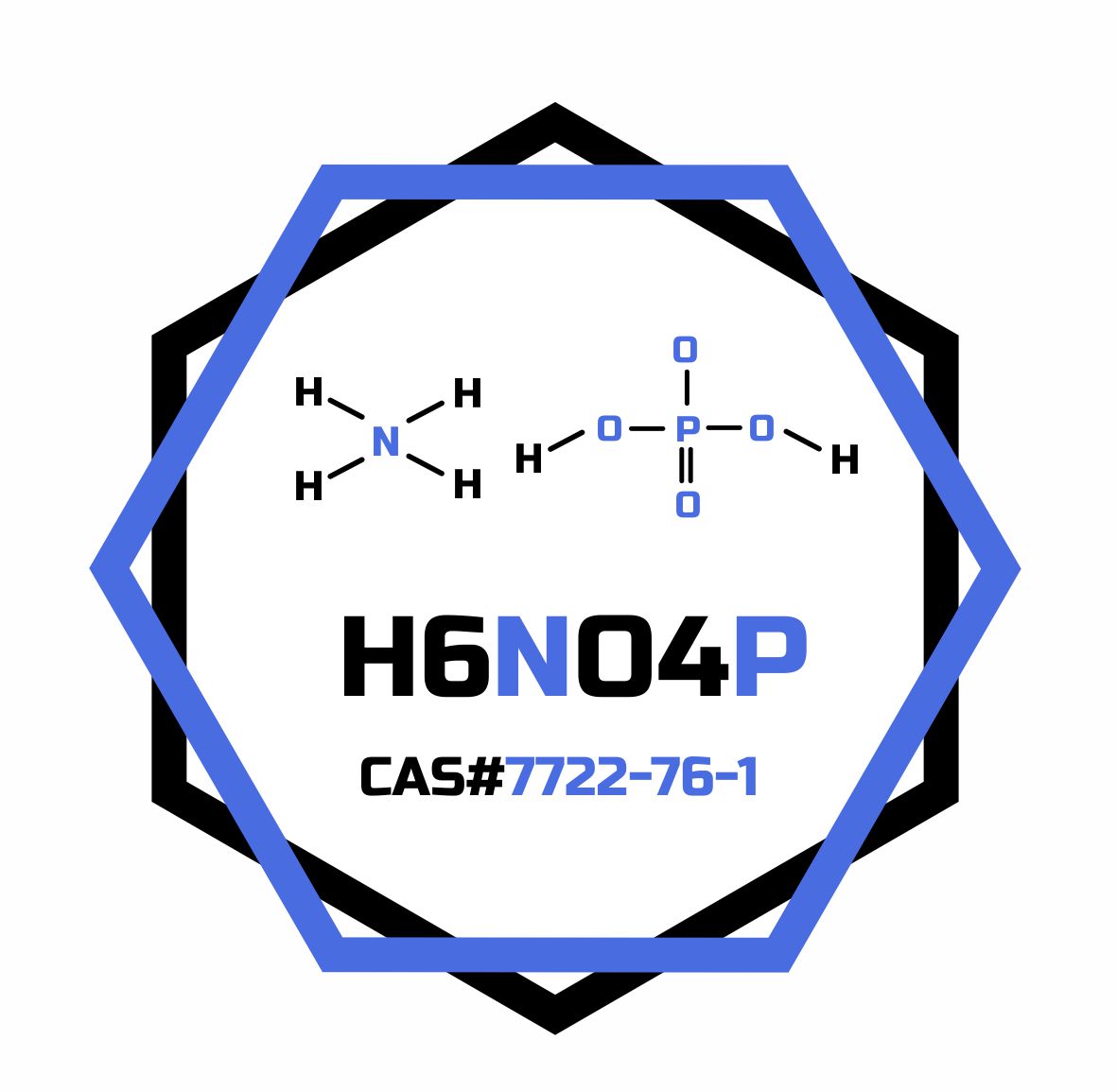
- Formula
- H6NO4P
- Molar mass
- 115.025 g/mol
- CAS Number
- 7722-76-1
- Density
- 1.81 g/cm3 at 20°C (68°F) – OECD Test Guideline 109
- Purity/Grade
- ≥98% (ACS Reagent)
- Apearance
- White crystalline
- Vapour pressure
- <0.00001 hPa (<0.00001 mmHg) at 20°C (68°F) – OECD Test Guideline 104
- Water solubility
- Soluble
- Melting point/freezing point
- 190°C (374°F) – dec.
- pH
- 3.8 – 4.4 at 50 g/l at 25°C (77°F)
Reviews
There are no reviews yet.